Executive Summary
Euformatics and Analityk Genetyka have collaborated successfully on the Polish market to sign several hospitals as Genomics Hub customers during 2023 and 2024. A number of genetic laboratories at public hospitals have been or are being onboarded to analyze clinical NGS data from locally collected samples. For more than a decade, Analityk Genetyka has been providing comprehensive support to users of Illumina NGS systems in Poland, which has resulted in the majority of sequencing data being generated on these platforms. Given the installed base of NGS instruments in Poland there is a significant upside potential to further expand the collaboration.
1. Background and Context
Euformatics develops and markets software tools for clinical genomic data analysis by hospitals and clinical laboratories. The tools are used for diagnosing rare diseases and cancer patients. The company was founded in 2010 and has operated profitably for most of its history, not having to rely on external investors to fund its operations.
Analityk Genetyka (AG) was founded in 2018, based on former OpenExome (2012-2018) company resources. AG from the very beginning is bonded with Analityk company creating joined structure called the Analityk Group. Analityk Genetyka provides complex portfolio including laboratory equipment, automatization, accessories, consumables, reagents and analysis software. Our mission is to deliver a comprehensive, NGS-based end-to-end solution to our customers. Many years of experience and productive cooperation both with our foreign partners and Polish customers shape the main pillar of our business.
The reason Euformatics and Analityk Genetyka decided to partner was clear – both companies were targeting the same end users in the clinical genomics space. Having an end-to-end offering from reagents and instruments to the tools needed to analyze genomic data and produce professional reports was important for Analityk Genetyka to serve their customers throughout the lab workflow. Euformatics, on the other hand, needed a local partner who had existing relationships and trust established with the local key opinion leaders and major medical institutions. The clinical genomics market in Poland is undergoing a significant transformation, driven by advancements in technology, increasing awareness of precision medicine, and supportive governmental and academic initiatives. Stakeholders must navigate issues such as funding, workforce development, and ethical concerns while capitalizing on advancements in technology and a supportive policy environment. By addressing these barriers, Poland can emerge as a leader in genomics-driven healthcare within Central and Eastern Europe, setting an example for neighboring markets.
2. The Partnership Strategy
The channel partnership started quite typically with product training and joint local event participation. Getting a new service known on a local market takes time and effort. Trust and perception of reliability and responsiveness by a service provider does not happen overnight. Clinical genomics in particular requires a high level of confidence in the analytical performance of the solutions because the end user is analyzing molecular level events. Those cannot be easily validated with other means so the performance of the kit, instrument and bioinformatics pipeline need to be on a high level. Often this means that validation is done separately for each customer to ensure that their patients are diagnosed using high quality data. This is what Euformatics and Analityk Genetyka set out to do with the labs that onboarded to Genomics Hub.
One such lab where we worked closely together with the geneticist team of the customer was Pomeranian Medical University in Szczecin (PUM).

Dr. Wojciech Kluźniak from PUM Szczecin comments on the collaboration: ”Our joint work with Euformatics and Analityk Genetyka has been very successful and allows us to effectively carry out NGS analyses. Euformatics provides advanced bioinformatics tools that enable precise and efficient analysis of sequencing data. Meanwhile, Analityk Genetyka supplies high-quality equipment and reagents necessary for laboratory research. The combination of solutions from both companies offers solid support for our projects and translates into the high quality of the results obtained. We assess the cooperation as professional, effective, and worth continuing. We especially appreciate the excellent support provided by the staff, whose professionalism and commitment greatly facilitate our work.”
3. Joint Market Engagement
Euformatics and Analityk Genetyka have jointly organized and participated in both physical and online events for the Polish clinical NGS market. Analityk Genetyka sponsored an event by the International School of Molecular Biology in 2022 in Warsaw at Lazarski University that brought together the key opinion leaders in the Polish market. Euformatics participated in demonstrating its clinical NGS data analysis tools for the participants.
We have also organized joint webinars for the Polish customers to introduce and onboard local laboratories to the Genomics Hub product family. The discontinuation of a software tool previously used in local laboratories marked a pivotal moment, as it prompted increased interest from labs seeking a suitable replacement. This resulted in multiple new users for Euformatics Genomics Hub in Poland.
4. Challenges and How They Were Overcome
The Polish labs that are starting out their NGS journey face the same challenges as labs elsewhere. Located in the EU, they need to be compliant with IVDR requirements and work with solutions that are either CE-IVD or IVDR certified. Assay selection, validation and verification is another area that requires effort. Analityk Genetyka can support their customers with the wet lab side activities while the application scientists at Euformatics onboard customers with NGS test validation in the beginning of the collaboration. These are important steps to ensure that patients are diagnosed based on high quality sequence data.
All new Genomics Hub customers from Poland already had their own quality management systems in place. The NGS operations needed to be added to those and full workflows documented properly. This has been a joint effort between the labs, Analityk Genetyka and Euformatics.

Dr. Agnieszka Sulewska from Olsztyn Children’s Hospital comments on the onboarding support: ”The teams at Analityk Genetyka and Euformatics have been instrumental in getting new tests introduced to our lab. They have supported us with the test validation and identification of problematic areas in the patient genome. These are important to detect as they could carry variants that do not get detected but are of clinical significance.”
Dr. Christophe Roos, Chief Scientific Officer at Euformatics continues: ”There is a tremendous and general drive to raise the ambition level of genetic diagnostics at Polish hospitals and laboratories. This is reflected in the fact that we have been working with very motivated people interested in taking 2768some steps deeper in the understanding of the complexity of NGS-based sequencing. This attention to details bodes well in a world where some products are presented under their ideal conditions, creating an illusion of simplicity. Knowing the difficulties and potential pitfalls removes risks from the patient care.”

5. Conclusions
Onboarding six new NGS sites in Poland for the Euformatics Genomics Hub has been a nice collaborative effort with Analityk Genetyka. AG has supplied consumables and instruments while Euformatics has taken the lead in setting up the bioinformatics pipelines for the customers and training local geneticists on how to automate diagnosis. The best evidence of successful onboarding has been the growing number of samples that are being analyzed with the Genomics Hub Each of the laboratories started with just a few samples per month, but now they use Genomics Hub routinely.
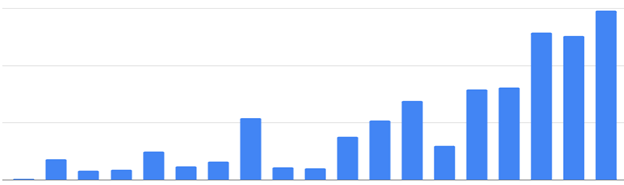
This is a great start to a collaboration we expect to continue and grow for years to come. The leadership of both companies is committed to maintaining a high level of service to Polish hospitals and clinical labs.

Tommi Kaasalainen, CEO of Euformatics comments: ”Analityk Genetyka is an example of a great local distributor for us. They know the clinical genetics space in Poland very well and have obtained the trust of the customers especially in the NGS area through their Illumina partnership. From our perspective, we could not have hoped for a better partner in Poland and we are looking forward to working with them to transition Poland more deeply into precision medicine.”
Dr. Juliusz Unrug, Head of Marketing and Market Access at Analityk Genetyka concludes: ”The collaboration with Euformatics represents a model example of implementing advanced solutions in the genetic testing market, providing full support to users in Poland. Through access to reliable data and comprehensive analyses, we are able to ensure consistent and effective support for all users of these solutions.”




